Austria-Based Biotech Firm’s Drug Shows Promise To Fight Covid-19, Gets Approval For Phase II Clinical Trial
An experimental drug developed by Austria-based Apeiron Biologics has been found to effectively block the cellular door SARS-CoV-2 uses to infect its hosts, says a new study.
The study, published in the journal Cell, provides new insights into key aspects of SARS-CoV-2, the virus that causes COVID-19, and its interactions on a cellular level, as well as how the virus can infect blood vessels and kidneys.
"We are hopeful our results have implications for the development of a novel drug for the treatment of this unprecedented pandemic," said lead researcher Josef Penninger Penninger, Professor at University of British Columbia in Canada.
"Our new study provides very much needed direct evidence that a drug -- called APN01 (human recombinant soluble angiotensin-converting enzyme 2 - hrsACE2) -- soon to be tested in clinical trials by the European biotech company Apeiron Biologics, is useful as an antiviral therapy for COVID-19," said Art Slutsky, Professor at the University of Toronto who is a collaborator on the study.
Apeiron Biologics on Thursday (2 March) announced that it has received regulatory approvals in Austria, Germany and Denmark to initiate a Phase II clinical trial of APN01 to treat COVID-19.
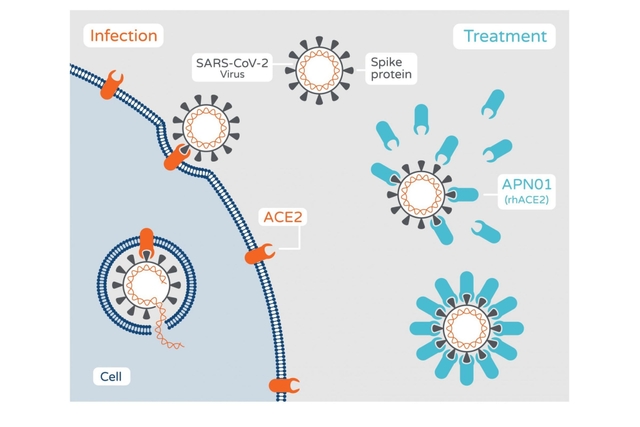
APN01 is the recombinant form of the human angiotensin-converting enzyme 2 (rhACE2), and has the potential to block the infection of cells by the novel SARS-CoV-2 virus, and reduce lung injury.
The Phase II trial aims to treat 200 severely infected COVID-19 patients, and the first patients are expected to be dosed shortly.
APN01 has a unique dual mode of action. APN01 imitates the human enzyme ACE2, which is used by the virus to enter cells.
The virus binds to soluble ACE2/APN01, instead of ACE2 on the cell surface, which means that the virus can no longer infect the cells.
At the same time, APN01 reduces the harmful inflammatory reactions in the lungs and protects against acute lung injury (ALI/acute respiratory distress syndrome (ARDS).
"Based on its unique dual mechanism of action, APN01 has the potential to be the first drug approved to treat COVID-19 that specifically targets the new SARS-CoV-2 virus," said Peter Llewellyn-Davies, Chief Executive Officer of Apeiron Biologics.
The randomized, double-blind Phase II trial will compare APN01 to placebo in up to 200 patients at 10 sites in Austria, Denmark and Germany. The primary objective of the trial is to assess the clinical efficacy and safety of APN01 in severe COVID-19 patients using, among other criteria, the need for invasive mechanical ventilation. Secondary objectives include the evaluation of measurable biological biomarker changes following treatment with APN01, the company said in a statement.
APN01 has been shown to be safe and well-tolerated in a total of 89 healthy volunteers and patients with pulmonary arterial hypertension (PAH) and ALI/ARDS in previously completed Phase I and Phase II clinical trials, it added.
(With inputs from IANS)